
In text Questions page number 25 Chapter 2: Acids, Bases and Salts Science Class 10 solutions are avalable at ourwebsite to help the students. In text Questions page number 25 is solved by our expert teachers. You can get ncert solutions and notes for class 10 chapter 2 absolutely free. NCERT Solutions for class 10 Science Chapter 2: Acids, Bases and Salts will definitely help in improving your marks in CBSE Board examinations.
In text Questions page number 25
Question 1. Why do HCl, HNO3, etc., show acidic characters in aqueous solutions while solutions of compounds like alcohol and glucose do not show acidic character?
Answer: When HCl or HNO3 are mixed with water then they dissolve in water to form H+ or H3O+ ions which shows their acidic character. For example just see the following reactions
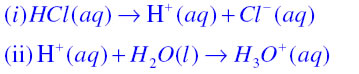
When alcohols and glucose are mixed with water then they do not dissolve to form ions. Hence they do not show acidic character.
Question 2. Why does an aqueous solution of an acid conduct electricity?
Answer: The presence of hydrogen (H+) or hydronium (H3O+) ions in the aqueous solution of an acid are responsible for conducting electricity.
Question 3. Why does dry HCl gas not change the colour of the dry litmus paper?
Answer: The colour of litmus paper changes only in the presence of ions like hydrogen (H+) or hydronium (H3O+) ions. HCl can produce these ions only in the form of aqueous solution. Hence dry HCl gas does not change the colour of dry litmus paper.
Question 4. While diluting an acid, why is it recommended that the acid should be added to water and not water to the acid?
Answer: The process of adding water to an acid is highly exothermic. If it is not done in the proper way then may the mixture splash out and causes serious burns. Therefore it is recommended that acid should be added to water carefully.
Question 5. How is the concentration of hydronium ions (H3O+) affected when a solution of an acid is diluted?
Answer: When the solution of an acid is diluted then its strength decreases as there is decrease in the concentration of hydronium (H3O+) ions.
Question 6. How is the concentration of hydroxide ions (OH-) affected when excess base is dissolved in a solution of sodium hydroxide?
Answer: When excess base is dissolved in the solution of sodium hydroxide then the concentration of hydroxide (OH-) ions increases.
Copyright @ ncerthelp.com A free educational website for CBSE, ICSE and UP board.